

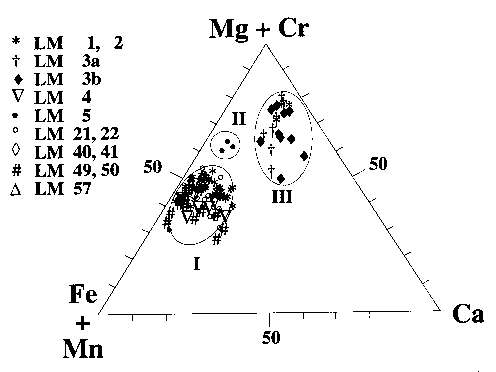
The findings also suggest the precipitation regions of a product may span a range of pH of aqueous solution, making it possible to simultaneously control pH and maintain the precipitation amount of the phase. Katoite and AFm phases form at Al 2O 3/CaO = ~3.0 under Si-deficient conditions. Strätlingite mainly occurs at intermediate levels of Si, Al and Ca. This part of the large triangular coordinate is adapted from ref. Schematic isothermal ternary phase diagram of PVDF/DMF/H 2 O.
The analytical results are in good agreement with the available experimental observations that the main precipitation regions of C-(N-)A-S-H appear at CaO/SiO 2 = ~1.0 and relatively low Al regions while N-A-S-H phases generally dominate the Ca-deficient regions of the contour diagrams. The roughness and microstructure of the films are characterized by atomic force microscopy (AFM) and scanning electron microscopy (SEM) measurements. The stability regions and quantities of simulated phases were identified in the SiO 2-CaO-Al 2O 3 ternary contour diagrams, yielding the overall relationships between the chemical components of precursors, phase assemblages and pH in pore solution.
#Afm ternary diagram free#
Gibbs free energy minimization method was conducted on AAMs spanning the compositional envelopes at (metastable) thermodynamic equilibrium. This study presents an analytical investigation based on thermodynamic simulation aimed at achieving a holistic management of the phase assemblages of alkali-activated materials (AAMs) and gaining insights into the designing of precursors.


 0 kommentar(er)
0 kommentar(er)
